
 SmartFilm ® Technology’s 허가완료제품
SmartFilm ® Technology’s 허가완료제품
| 제품명 | 허가일 | 구분 | 성분 | 효능/효과 |
| 비아그라엘구강붕해필름50밀리그램 | 2012/05/11 | ETC | 실데나필 시트르산염 |
발기부전의 치료 |
| 비아그라엘구강붕해필름100밀리그램 | 2012/05/11 | ETC | 발기부전의 치료 | |
| 불티스구강붕해필름25 | 2014/02/12 | ETC | 발기부전의 치료 | |
| 아트페질구강붕해필름10 | 2013/11/29 | ETC | 도네페질염산염일수화물 | 알츠하이머형 치매증상의 치료 |
| 아트페질구강붕해필름5 | 2014/02/28 | ETC | 알츠하이머형 치매증상의 치료 | |
| 불티움구강붕해필름10 | 2014/11/28 | ETC | 타다라필 | 발기부전(erectile dysfunction)의 치료 |
| 불티움구강붕해필름20 | 2014/11/05 | ETC | 발기부전(erectile dysfunction)의 치료 | |
| 타민비구강붕해필름 | 2018/06/21 | OTC | 피리독신염산염등 | 구각, 구순, 구내염, 설염, 습진, 피부염, 임신수유기,
병중, 병후 체력 저하 시 |
| 오비케어구강붕해필름10 | 2018/07/19 | ETC | 솔리페나신숙산산염 | 절박성 뇨실금, 빈뇨, 요절박과 같은 과민성 방광
증상의 치료 |
| 국내사 제공 품목 | ||||
| 비아그라엘 구강붕해필름 50밀리그램, 100 |
2012/05/11 | ETC | 실데나필 시트르산염 |
발기부전의 치료 |
| 타오르구강붕해필름(대웅제약) 5, 10, 20 |
2015/05/27 | ETC | 타다라필 | 발기부전(erectile dysfunction)의 치료 |
| 해외 수출 품목 | ||||
| 인도네시아 | CIASTAR ODF 10,20mg (Sildenfil citrate), VIASTAR ODF 50,100mg (Sildenfil citrate) | |||
| 페루 | Vulteim ODF 20mg (Tadalafil) | |||
| 모로코외 아프리카지역 | Vector ODF 20mg (Tadalafil) | |||
| 기타 | 베트남 (1번 선적), 필리핀, 대만, 페루 등 현 제품 등록중 | |||
 SmartFilm ® Technology’s 해외특허현황
SmartFilm ® Technology’s 해외특허현황
| 제품명 | 특허명칭 | |||||
| 실데나필스트레이트 ODF |
High-content fast dissolving film with masking of bitter taste comprising sildenafil as active ingredient (PCT-KR2013-001679, 2013.02.28) |
|||||
| 개별국 진입 | 출원번호 | 출원일 | 등록번호 | 등록일 | ||
| 1 | 미국 | 14/379,989 | 2014/08/20 | US10,092,651 | 2018/10/09 | |
| 2~5 | 유럽 4개국 (독일, 이태리, 스위스, 스웨덴) |
13755861.5 | 2014/09/26 | DE602013036508.3 | 2018/07/19 | |
| IT502018000022055 | 2018/07/24 | |||||
| 13755861 | 2018/07/25 | |||||
| SE2821066T3 | 2018/07/24 | |||||
| 6 | 캐나다 | 2864322 | 2014/08/11 | 2,864,322 | 2016/08/09 | |
| 7 | 일본 | 2014-554675 | 2014/07/28 | 5941558 | 2016/05/27 | |
| 8 | 중국 | 2013800107591 | 2014/08/22 | ZL2013800107591 | 2020/02/21 | |
| 9 | 인도 | 1706/MUMNP/2014 | 2014/08/25 | 333889 | 2020/03/04 | |
| 제품명 | 특허명칭 | |||||
| 타다라필 ODF |
Tadalafil oral dispersible film and preparing method thereof (PCT-KR2015-009151, 2015.08.31) |
|||||
| 개별국 진입 | 출원번호 | 출원일 | 등록번호 | 등록일 | ||
| 1 | 일본 | 2017-533147 | 2017/03/02 | 6417480 | 2018/10/12 | |
| 2 | 호주 | 2015312672 | 2017/03/02 | 2015312672 | 2018/08/23 | |
| 3 | 인도네시아 | P-00201701773 | 2017/03/20 | IDP000063356 | 2019/10/08 | |
| 4 | 유럽 3개국 (영국, 프랑스, 독일) |
15838561.7 | 2017/03/23 | UK EP3188720 | 2021/04/21 | |
| FR EP3188720 | ||||||
| DE 602015068468.0 | ||||||
| 5 | 중국 | 201580058055.0 | 2017/04/25 | ZL201580058055.0 | 2020/11/13 | |
| 6 | 홍콩 | 17109657.6 | 2017/09/22 | HK1235704 | 2021/04/01 | |
| 7 | 필리핀 | 1-2017-500388 | 2017/03/02 | 1-2017500388 | 2021/04/23 | |
| 8 | 베트남 | 1-2017-00884 | 2017/03/10 | 32918 | 2022/07/08 | |
| 9 | 사우디아라비아 | 517381018 | 2017/03/01 | 7993 | 2021/05/18 | |
 ODF 적용 카테고리
ODF 적용 카테고리
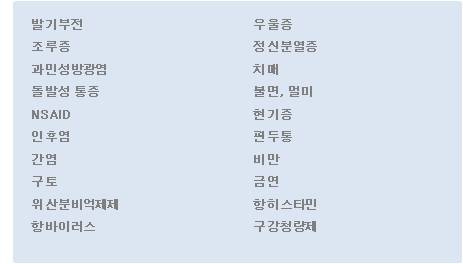
SmartFilm® Technology 는 잠정적으로 정신신경계 (Central nervous system), 즉 정신분열증치료제, 항우울제, 치매치료 및 예방제, ADHD치료제 등에 집중하려 하며, 편두통치료제, 항구토제나 기타 비뇨기과질환과 같은 필요영역에도 적용할 수 있습니다. 이 같은 치료영역을 포함하여 서울제약은 SmartFilm® Technology 를 필요로 하는 새로운 치료영역에 대해서도 파트너사와의 지속적인 협력을 통한 공동개발의 기회를 활짝 열어놓고 있습니다.
또한, 장기적인 기술의 심화 및 응용을 통해 Fast-onset 제품, 생물학적 백신, 성호르몬 제제 등 다양하고 고부가가치적인 제품을 개발하게 될 것 입니다.